INVERTASE from Candida sp.
IVH-101
| Appearance: | White amorphous powder, lyophilized | ||
|---|---|---|---|
| Activity: | GradeⅠ 100U/mg-solid or more (containing approx. 70% of stabilizer) |
||
| Stabilizer: | KH₂PO₄ | ||
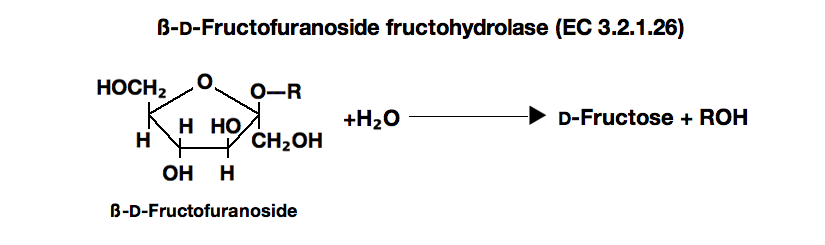
| Stability: | Stable at -20°C for at least One year (Fig.1) |
|---|---|
| Molecular weight : | approx. 260,000 |
| Michaelis constant : | 1.5×10⁻²M (Saccharose) |
| Structure : | Glycoprotein containing ca. 50% of carbohydrates |
| Optimum pH : | 3.5-4.0(Fig.3) |
| Optimum temperature : | 60-70°C(Fig.4) |
| pH Stability : | pH 4.0-6.0 (50°C, 10min)(Fig.5) |
| Thermal stability : | below 60°C (pH 4.5, 10min)(Fig.6) |
| Substrate specificity: | The enzyme hydrolyzes saccharose and raffinose, but does not hydrolyze inulin and melezitose. ⁶⁾ |
APPLICATIONS
This enzyme is useful for enzymatic determination of saccharose and for the structure investigation of carbohydrates containing ß-D-fructofuranoside residue.
ASSAY
Principle:
invertase
Saccharose+H₂O ► D-Fructose+D-Glucose
The appearance of reducing sugars is measured by the modified Fehling-Lehmann-Schoorl method. ⁷⁾
Unit definition:
One unit causes the formation of one milligram of reducing sugars equivalent to the glucose at 3 minutes under the conditions described below (This activity is equivalent to the international unit that hydrolyzes one micromole of saccharose per minute at the same temperature).
Method:
| A. Saccharose solution: | 5.0%[5.0g saccharose/100ml of 80mM acetate buffer, pH 4.5 (add 2-3 drops of toluene for preservation)] |
|---|---|
| B. Alkaline solution: | (103g NaOH, 346g Rochelle salt・4H₂O/1,000ml of H₂O) |
| C. CuSO₄ solution: | 6.93% (69.3g CuSO₄・5H₂O/1,000ml of H₂O) |
| D. KI solution: | 30% (300g KI/1,000ml of H₂O)(Store in a brownish bottle) |
| E. H₂SO₄ solution: | 25% |
| F. Na₂S₂O₃ solution: | 50mM (49.638g Na₂S₂O₃・5H₂O, 4.0g Na₂CO₃ (stabilizer)/4,000ml of H₂O)(Store in a brownish bottle and keep for 3-4 days before use) |
| G. Soluble starch solution: | 2.0% (Dissolve by boiling) (Should be prepared fresh) |
| H. Enzyme diluent: | 50mM acetate buffer, pH 4.5 |
Procedure
| Concentration in assay mixture | |
|---|---|
| Acetate buffer | 65mM |
| Saccharose | 73mM |
1. Pipette 1.0ml of substrate solution (A) into a test tube and equilibrate at 20°C for about 5 minutes.
2. Add 1.0ml of the enzyme solution (pre-incubated at 20°C) and mix.
3. After exactly 3 minutes at 20°C, add 2.0ml of alkaline solution (B) to stop the reaction. At the same time, prepare the blank by first mixing the substrate solution with 2.0ml of alkaline solution after a 3 min-incubation at 20°C, followed by the addition of the enzyme solution and carry out the same
procedure as the test (Procedure 4-8).
4. Transfer the stopped reaction mixture from the test tube to a 100ml volume of Erlenmeyer flask. Rinse the
inside of the test tube with about 3ml of distilled water and transfer the rinsings to the flask. Repeat this
procedure three times.
5. Add 2.0ml of CuSO₄ Solution (C) and place the flask directly on a electrothermic heater (1.2 KWH) in the
presence of a glass bead (5mmϕ) to prevent bumping.
6. Keep for exactly 2 minutes in a boiling state and cool down to room temperature under running water.
7. Add 2.0ml each of KI solution (D) and H₂SO₄ solution (E) in this order.
8. Shake the flask and determine the amount of residual Cu⁺⁺ by titration with Na₂S₂O₃ solution (F) in the
presence of a few drops of soluble starch (G) as an indicator.
9. Record the titers (ml) of the test (t) and the blank (b), and calculate the titration difference (Δtiter, ml).
* Dissolve the enzyme preparation in ice-cold distilled water and dilute to 2.0-9.0 U/ml with the enzyme dilute (H), immediately before assay.
Calculation
Activity can be calculated by using the following formula :

Δtiter (b-t)×F×df
Volume activity (U/ml) = = Δtiter×F×1.66×df
0.600×Vs
Weight activity (U/mg)=(U/ml)×1/C
- Vs
- : Sample volume (1.0ml)
- 0.600
- : Titration difference (ml) of 50mM Na₂S₂O₃ solution (F=1.00) for 1.0mg of reducing sugar (glucose)
- F
- : Concentration factor of 50mM Na₂S₂O₃ (F should be determined by the iodotimetry method in each time of the preparation).
- C
- : Enzyme concentration in dissolution (c mg/ml)
REFERENCES
- H.Negoro; J.Agric.Chem.Soc. (Japan), 31, 253 (1957).
- K.Myrbck; The Enzyme (2nd ed.) Vol. 14, p.379 (1960), AP.
- T.Kaya; J.Agr.Chem.Soc. (Japan), 38, 417 (1964).
- J.Hoshino and A.Momose; J.Gen.Appl.Microbiol., 12, 163 (1966).
- O.Lampen et el.; J.Biol.Chem., 243, 1573 (1968).
- H.Negoro; J.Ferment, Technol., 51, 879, (1973).
- T.Yamamoto, J.Kumada and T.Sawai; Bull.Agric.Biol.Chem.Soc. (Japan); 21, 185 (1957).
To get a quote, contact us at info@toyobousa.com, or INQUIRY.