LACTATE DEHYDROGENASE from Pig heart
| Appearance: | Crystalline suspension in 1.6M ammonium sulfate solution | ||
|---|---|---|---|
| Activity: | GradeⅡ 2,000U/ml or more | ||
| Contaminants: | Malate dehydrogenase ≤5.0×10⁻²% Pyruvate kinase ≤3.0×10⁻²% GPT ≤3.0×10⁻²% |
||
| Stabilizers: | NADH, 2-mercaptoethanol | ||
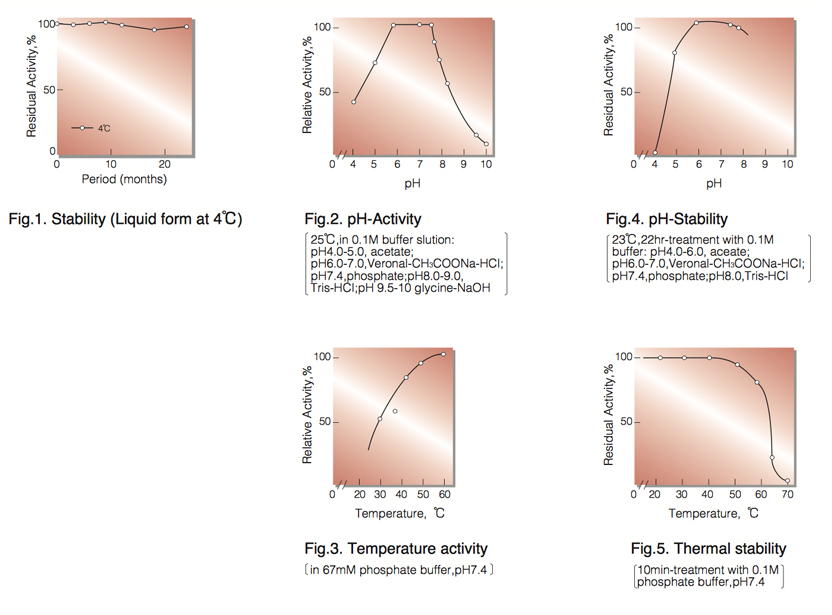
| Stability: | Stable at 4°C for at least one year(Fig.1) |
|---|---|
| Molecular weight : | 115,000±6,500 |
| Michaelis constants : | 2.5×10⁻²M (Lactate), 1.0×10⁻⁴M (Pyruvate) |
| Structure : | |
| Inhibitors : | I ‾, Ag⁺, Hg⁺⁺, p-chloromercuribenzoate, LDH inhibitors (formed from NADH) |
| Optimum pH : | 6.0-7.4(Fig.2) |
| Optimum temperature : | above 60°C(Fig.3) |
| pH Stability : | pH 6.0-8.0(23°C, 22hr)(Fig.4) |
| Thermal stability : | below 50°C(pH 7.4, 10min)(Fig.5) |
APPLICATIONS ⁴⁾
This enzyme is useful for enzymatic determination of numerous metabolites, e.g.ATP, ADP, glucose, creatinine, pyruvate, lactate and glycerol, and of enzyme activities, e.g.GPT, PK and CPK when coupled with the related enzymes.
LCD-209
ASSAY
Principle:
lactate dehydrogenase
Pyruvate+NADH+H⁺ ► L-Lactate+NAD⁺
The disappearance of NADH is measured at 340nm by spectrophotometry.
Unit definition:
One unit causes the oxidation of one micromole of NADH per minute under the conditions described below.
Method:
| A. Pyruvate solution: | 5.0mM sodium pyruvate (Should be prepared fresh) |
|---|---|
| B. K-Phosphate buffer, pH 7.4: | 1.0M |
| C. NADH solution: | 1.0mM (Should be prepared fresh) |
| D. Enzyme diluent: | 0.1M K-phosphate buffer, pH 7.4 contg. 0.1% of BSA |
Procedure
| Concentration in assay mixture | |
|---|---|
| K-Phosphate buffer | 67 mM |
| Pyruvate | 0.50 mM |
| NADH | 0.10 mM |
| BSA | 3.3µg/mM |
1. Immediately before use prepare the following working solution (10 tests) in a brownish bottle and store on ice.
3.0 ml Substrate solution (A)
2.0 ml K-Phosphate buffer, pH 7.4 (B)
3.0 ml NADH solution (C)
22..0 ml H₂O
2. Pipette 3.0ml of working solution into a cuvette (d=1.0cm) and equilibrate at 25°C for about 5 minutes.
3. Add 0.05ml of the enzyme solution* and mix by gentle inversion.
4. Record the decrease in optical density at 340nm against water for 2 to 3 minutes in a spectrophotometer
thermostated at 25°C, and calculate the ΔOD per minute from the initial linear portion of the curve (ΔOD test).
At the same time, measure the blank rate (ΔOD blank) by using the same method as the test except that the enzyme diluent is added instead of the enzyme solution.
* Dilute the enzyme preparation to 0.2-1.0U/ml with ice-cold enzyme diluent (D), immediately before assay.
Calculation
Activity can be calculated by using the following formula :

ΔOD/min (ΔOD test-ΔOD blank)×Vt×df
Volume activity (U/ml) = = ΔOD/min×9.81×df
6.22×1.0×Vs
Weight activity (U/mg)=(U/ml)×1/C
- Vt
- : Total volume (3.05ml)
- Vs
- : Sample volume (0.05ml)
- 6.22
- : Millimolar extinction coefficient of NADH (㎠/micromole)
- 1.0
- : Light path length (cm)
- df
- : Dilution factor
REFERENCES
- R.Jaenicke and G.Pfleiderer; Biochim.Biophys.Acta, 60, 615 (1962).
- J.J.Holbrook et al; The Enzymes, 11, 191 (1975).
- J.Everse and N.O.Kaplan; Adv.in Enzymol., 37, 61 (1973).
- C.A.Loshon, R.B.McComb, L.W.Bond, G.N.Bowers, Jr.W.H.Coleman and R.H.Gwynn; Clin.Chem., 23,1576 (1977).
To get a quote, contact us at info@toyobousa.com, or INQUIRY.
